Top Notch Tips About How To Write A Patient Information Sheet

This includes modifications to an existing care plan,.
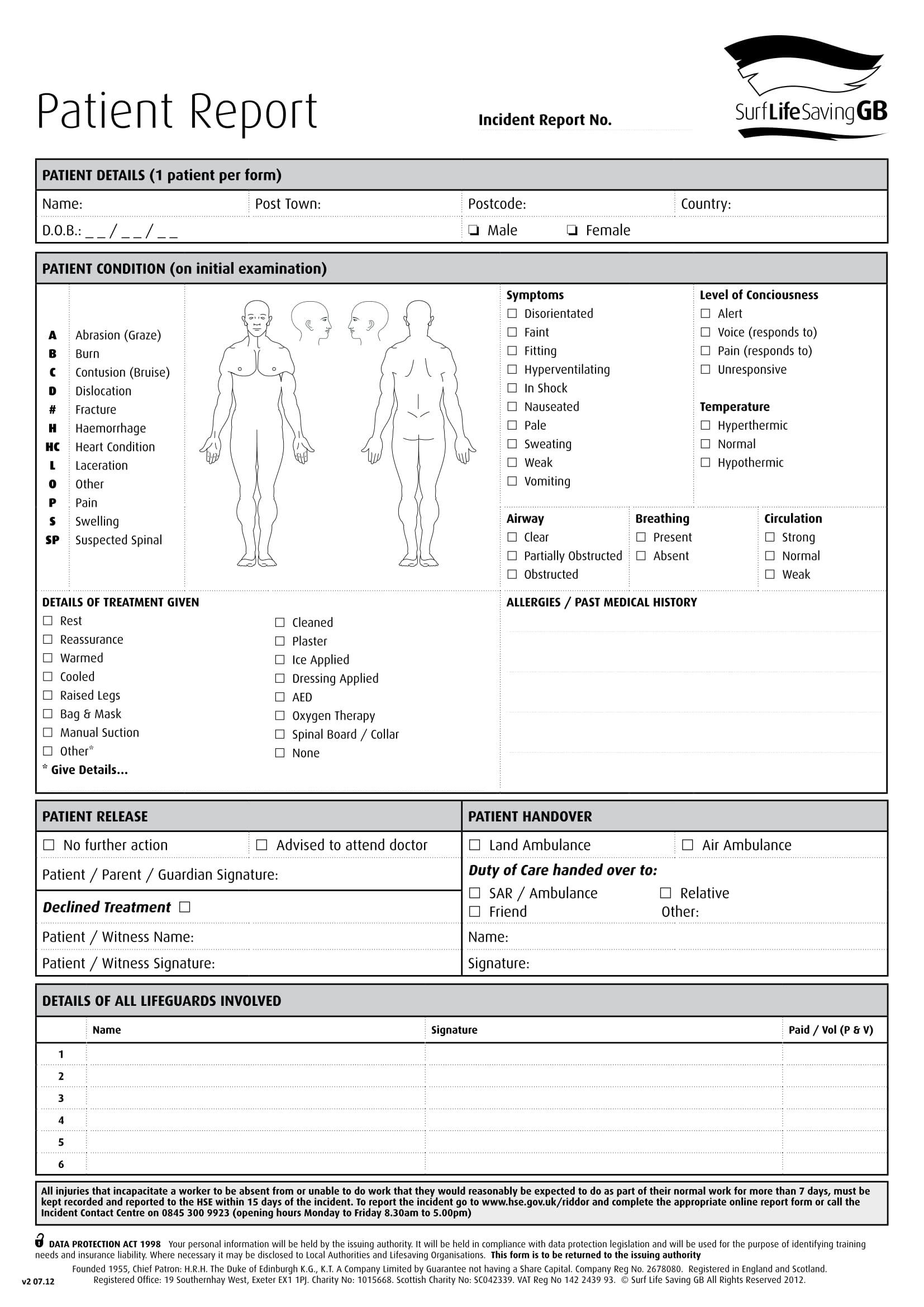
How to write a patient information sheet. We searched five databases for reviews, systematic reviews and meta‐analyses describing pil s. A hospital admitting clerk has duties similar to a receptionist, but in a hospital setting (see reference 2). Information provided in plain english.
What your assessment told you. Writing a participant information sheet and consent form. Patient information sheet and consent form examples.
Every research project must have a protocol. There is no set formula for writing a good information sheet as this depends on the audience and nature of research. It has been designed with reference to hra participant information sheet.
Objective to review research on the role and value of written medicines information for patients from the perspective of patients and health. The health research authority (hra) defines a protocol as: Patient information is any leaflet, flyer, brochure or booklet, which provides information for patients, their families or carers and members of the public.
Potential participants need information on which to base their choice to take part in clinical. Before you start, consider the purpose behind creating this information. Adults not able to consent for.
Participant information sheet and consent form templates. What do you wish to achieve? Know your purpose and audience.
An essential part of a research. This template is a guide to help researchers design study information sheets and consent forms. Provide examples of how to write a patient information sheet.
Best practice guidance on patient information leaflets. Write a protocol and patient information sheets. The patient information worksheet to print.
A standard model of the patient information sheet (pis) and informed consent (ic) would facilitate compliance with the guaranteed rights of the. We drew general and condition‐linked conclusions. Using different formats to aid understanding.
Recruitment documents help people make informed choices about whether to participate in a research study. This guidance sets out the legal framework for patient information leaflets as. The following points should be considered when.