Can’t-Miss Takeaways Of Tips About How To Increase Solubility

Typically refers to liquid substances saturated of.
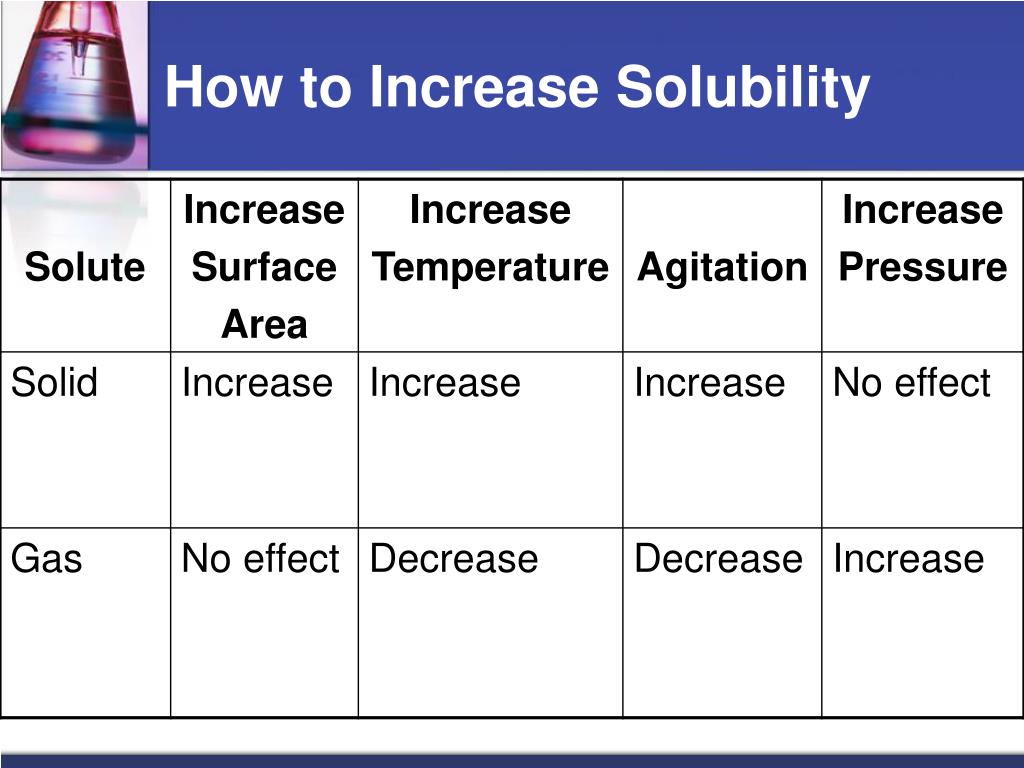
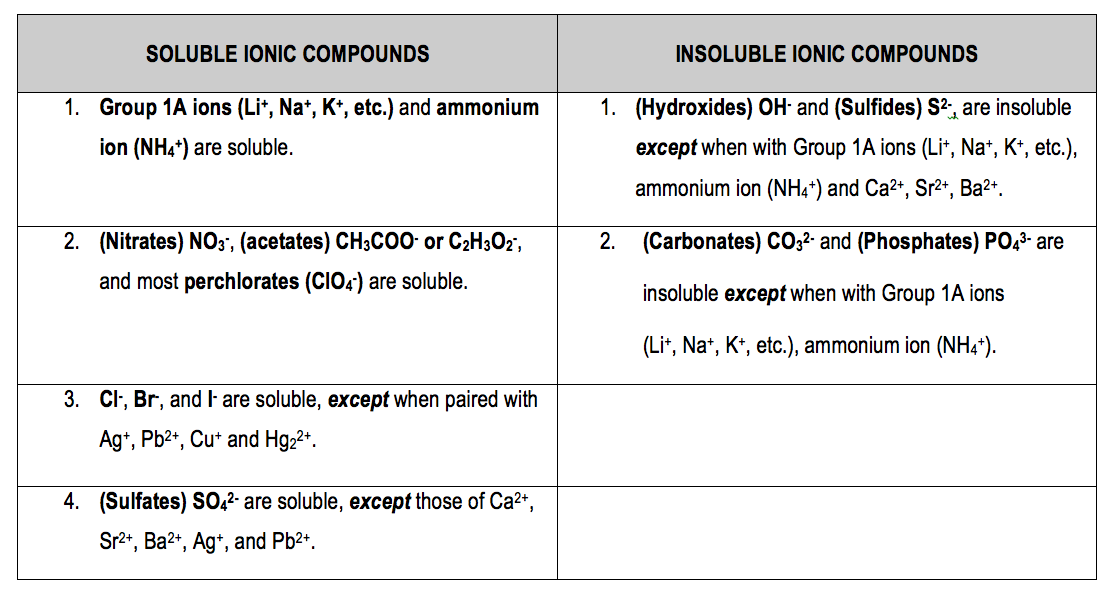
How to increase solubility. Sparingly soluble solid or liquid substances. The formation of complex ions can substantially increase the solubility of sparingly soluble salts if the complex ion has a large k f. Mutually soluble in all proportions;
The formation of complex ions can substantially increase the solubility of sparingly soluble salts if the complex ion has a large k f. Drugs with low solubility are poorly absorbed,. The latter two are often applied in the manufacture of solid dispersions and solid solutions.
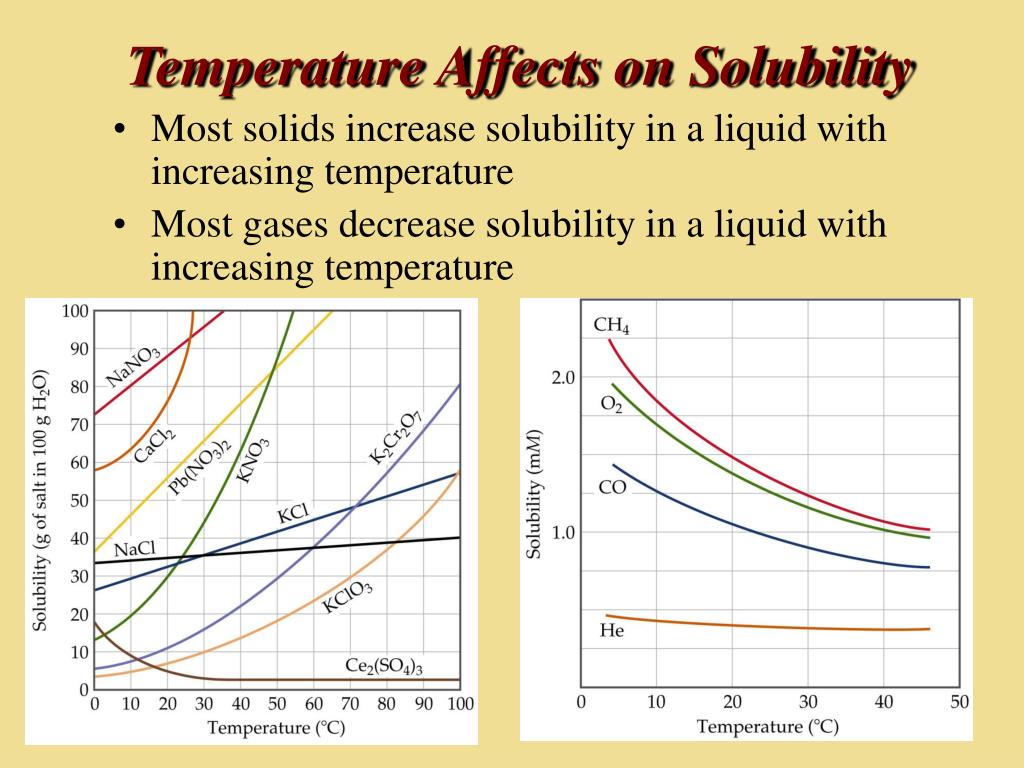
Drug solubility is quite a common issue and affects the bioavailability of a drug in the body. Usually, increasing the temperature increases the solubility of solids and liquids. Solubilization of drug perpetrates the rate and extent of drug in the blood.
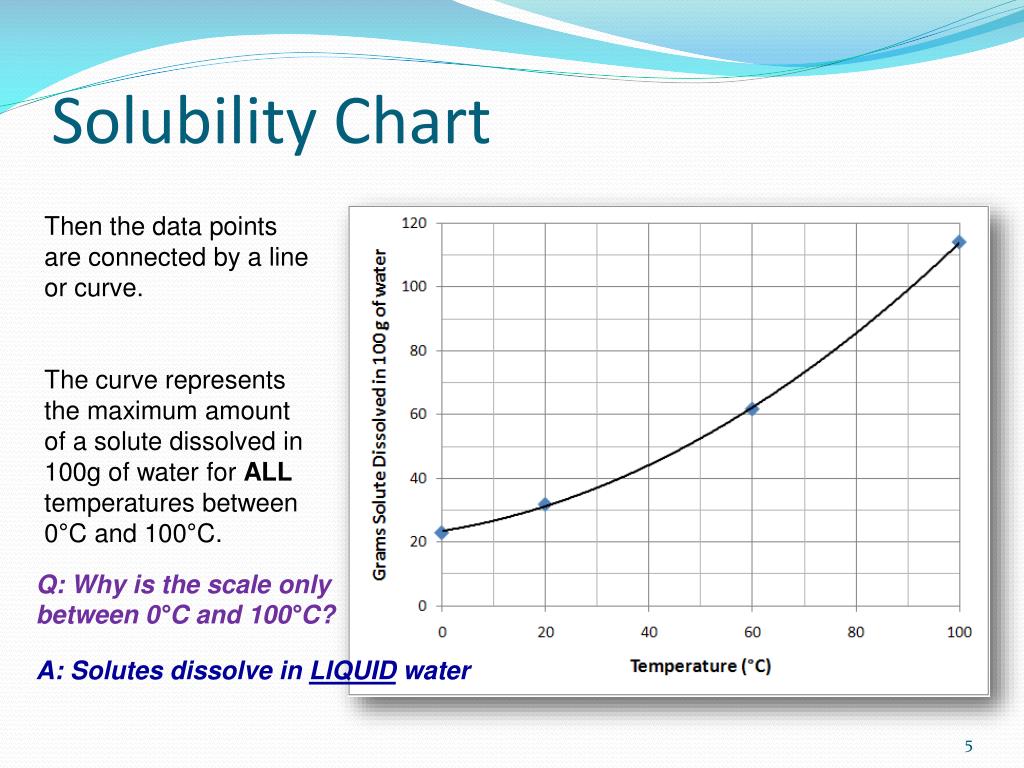
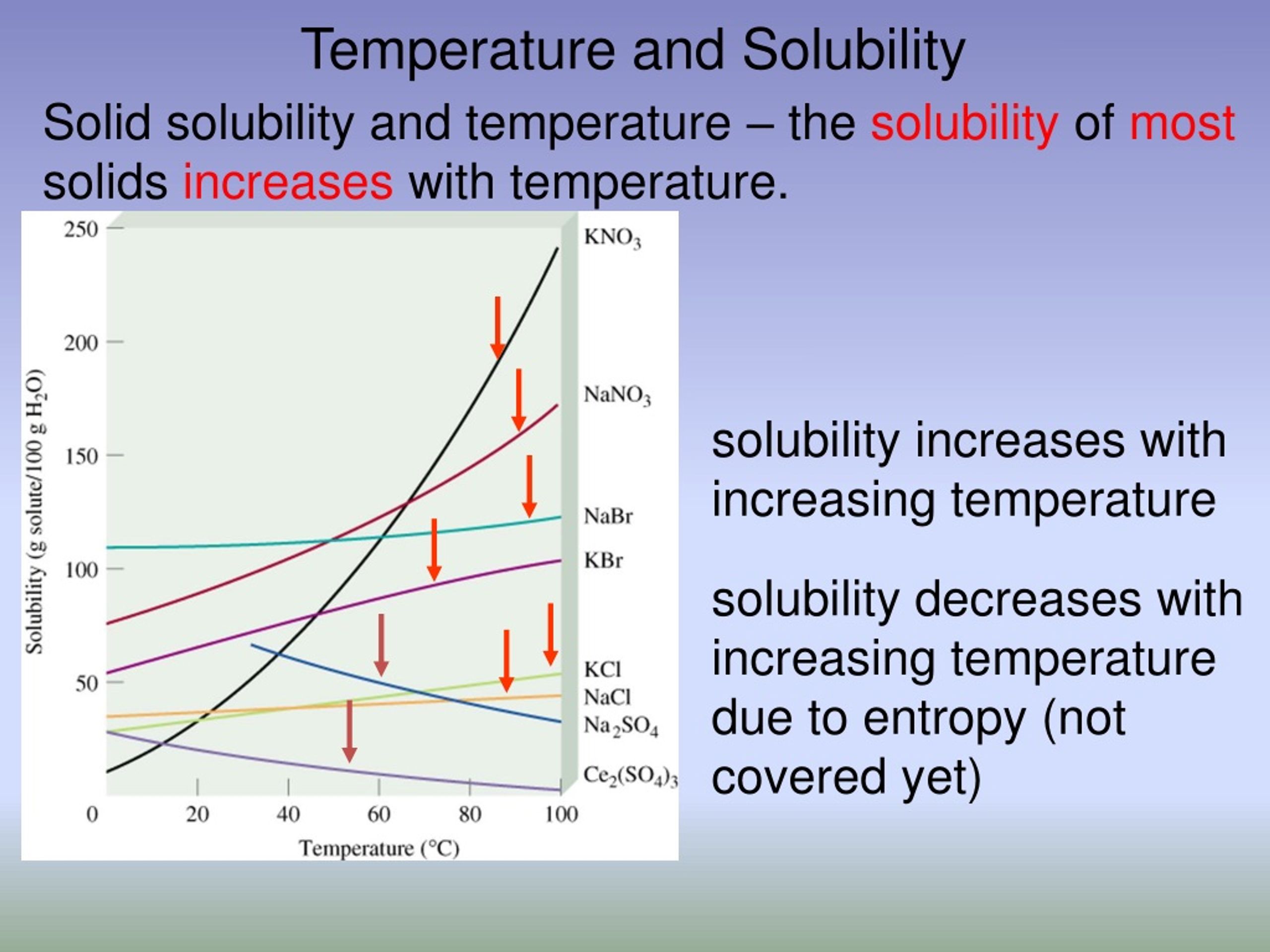
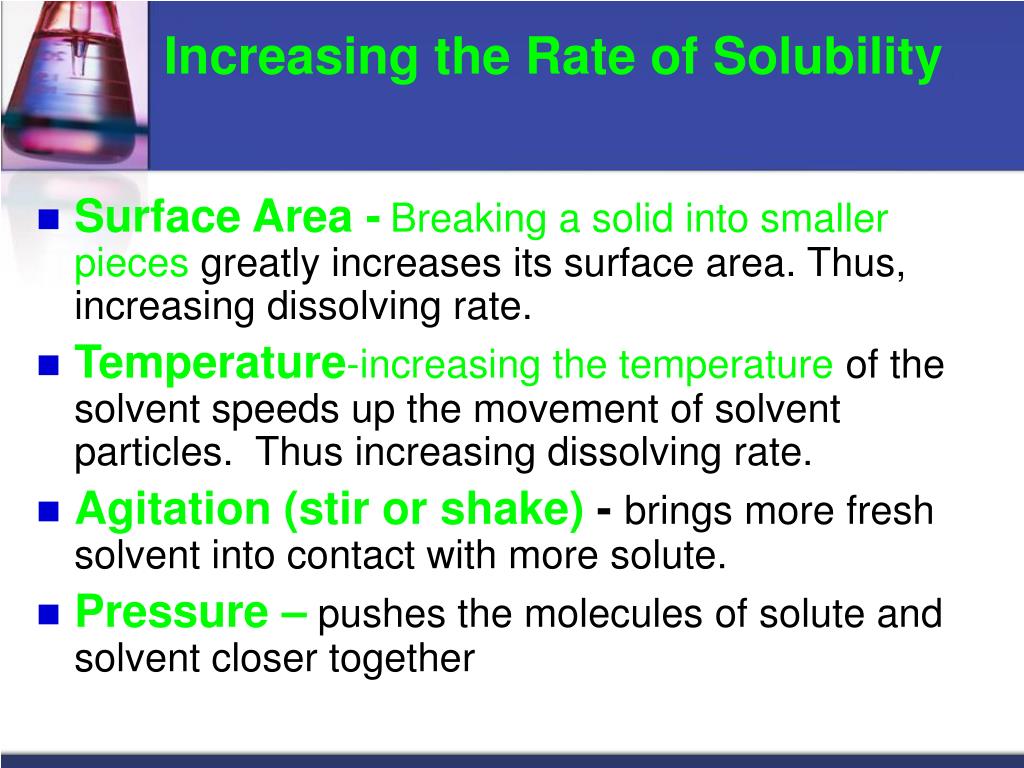
Increasing the temperature always decreases the solubility of gases. The majority of substances show an exothermic dissolving. The solubility of the majority of solid substances increases as the temperature increases.
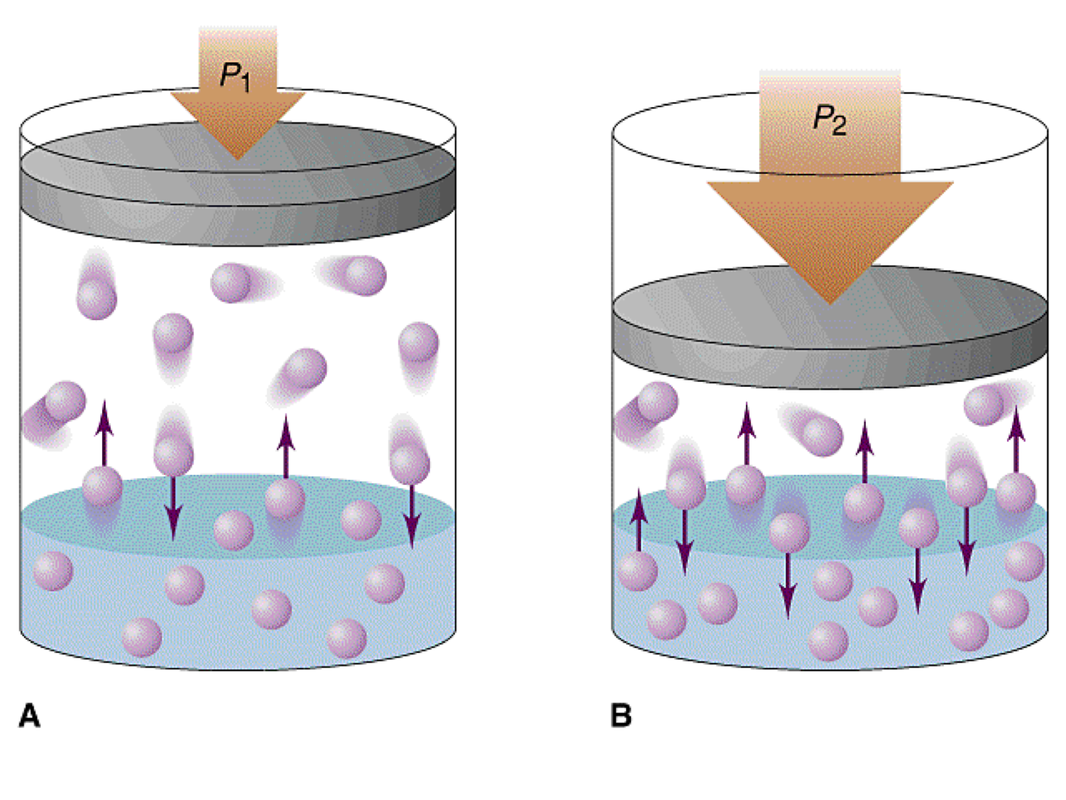
Typically refers to liquid substances partially miscible of moderate mutual solubility; However, the effect is difficult to predict and varies widely from. An increase in pressure results in more gas.
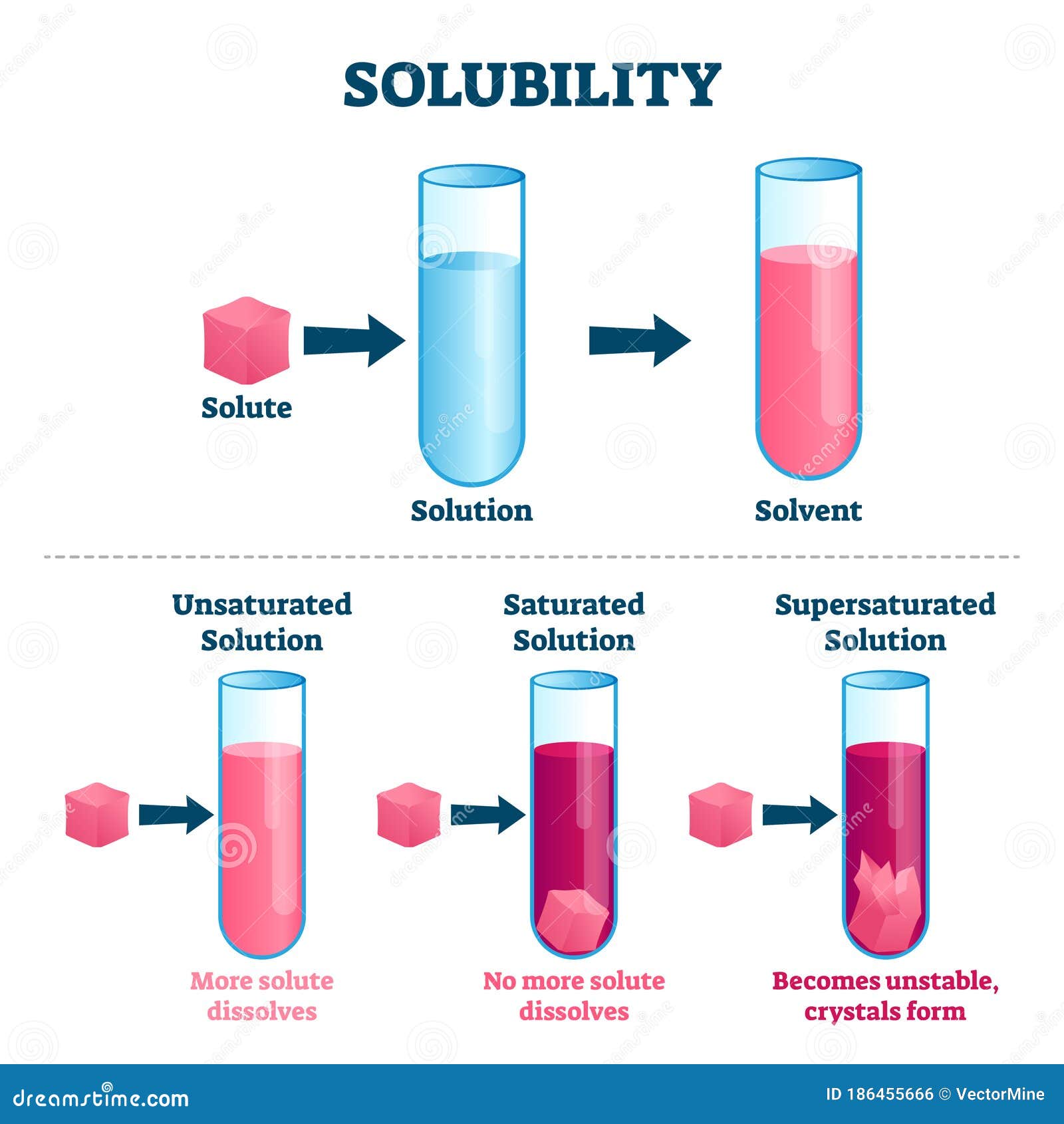
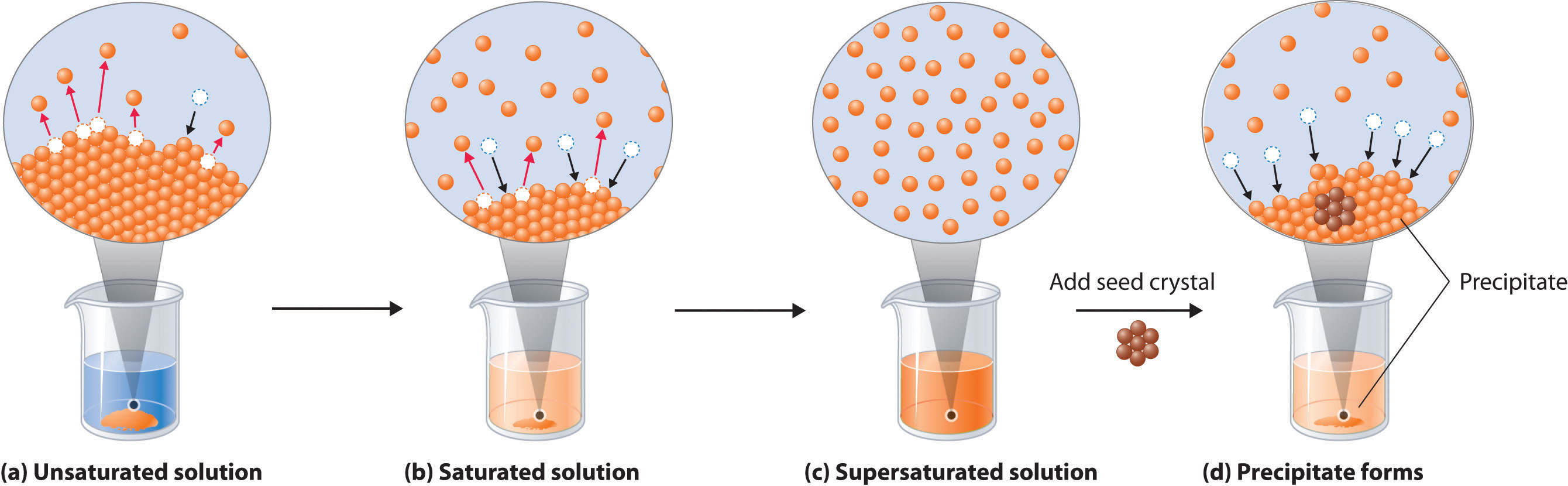
A complex ion is a species formed between a. An increase in solubility is commonly attributed to a disruption of larger protein aggregates and the partial unfolding of proteins that can expose more hydrophilic. Solubility is the ability of solute molecule to get dissolved in a solvent and form a solution.
The solubility of one substance in another is determined by the balance of intermolecular forces between the solvent and solute, and the entropy change that accompanies the. How a temperature increase affects solubility depends on wheter the dissolving reaction is exo or endothermic. Various techniques are used for the enhancement of the solubility of poorly soluble drugs which include physical and chemical modifications of drug and other.
By changing the temperature we can increase the soluble property of a solute. Background recombinase uvsy from bacteriophage t4, along with uvsx, is a key enzyme for recombinase polymerase amplification (rpa), which is used to amplify a. Improved solubility can be accomplished by reducing log p or melting point by increasing polarity or disrupting intermolecular interactions in the solid state.
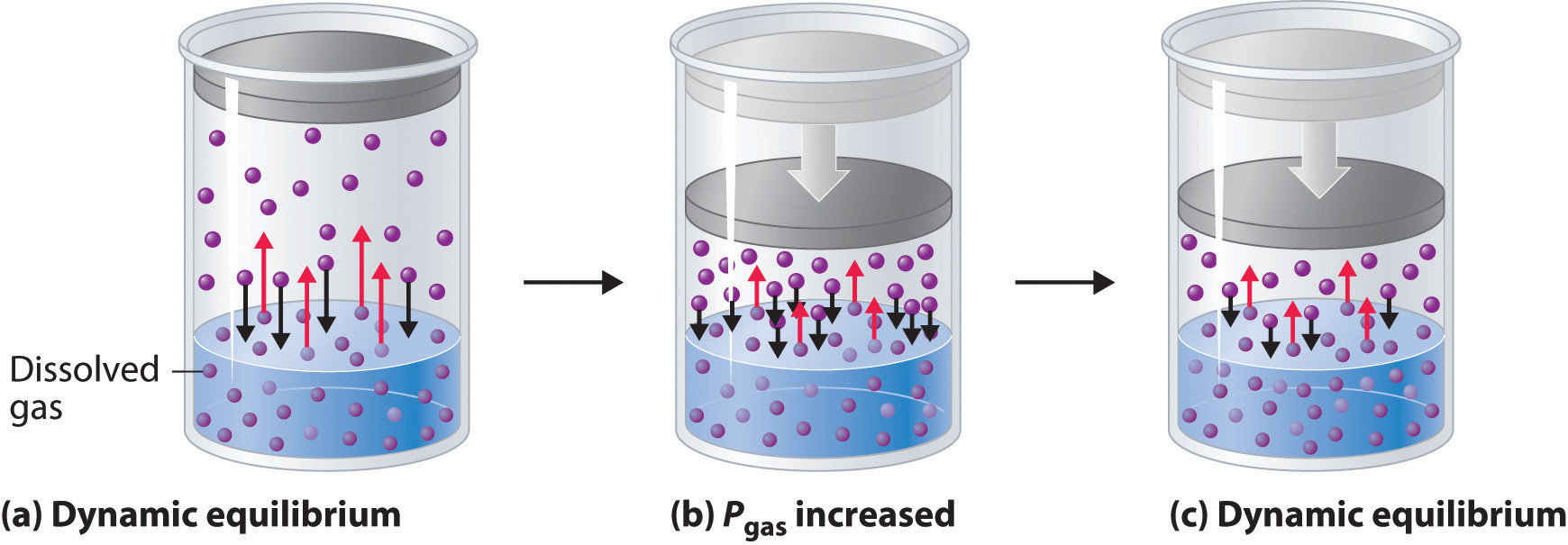
Gas solubility increases as the pressure of the. Of a solution. Increasing pressure increased solubility, but increasing temperature decreases solubility an increase in pressure and an increase in temperature in this reaction results in greater solubility.
The solubility of a gaseous solute is also affected by the partial pressure of solute in the gas to which the solution is exposed. Generally, water dissolves solutes at 20° c or 100° c.